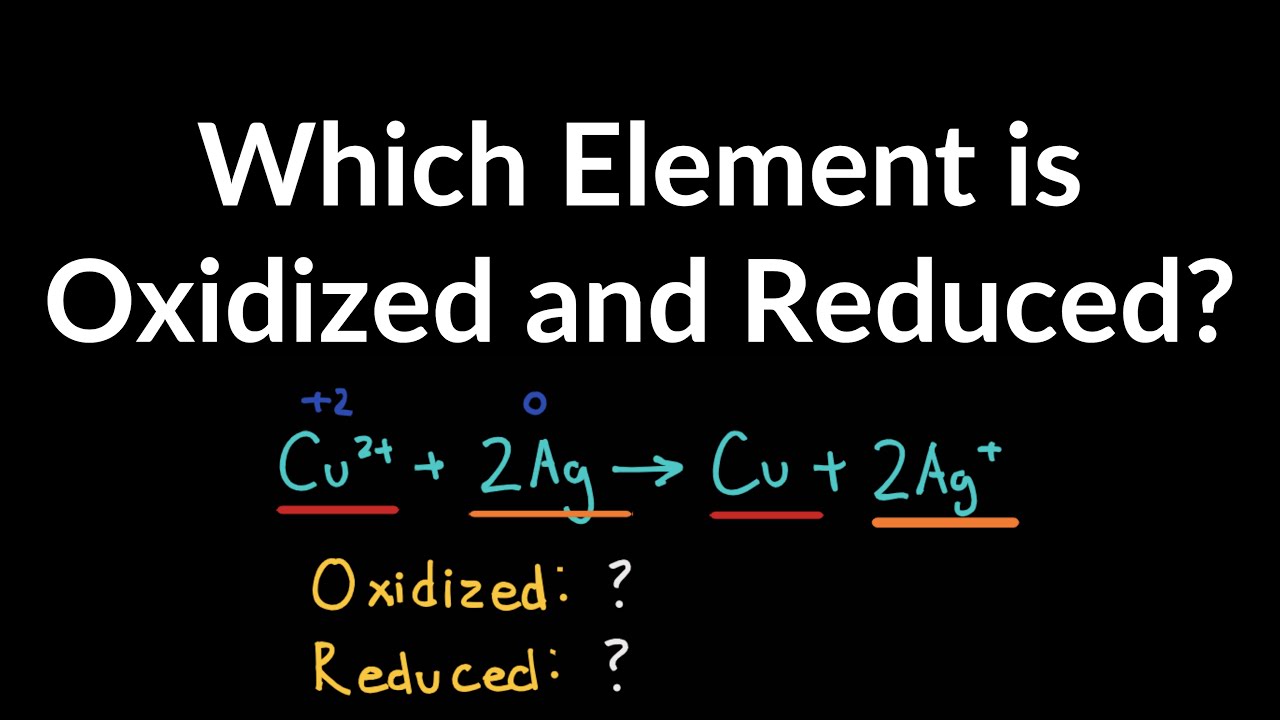
Which Element Is Oxidized in the Reaction Below
The oxidation state which describes the degree of. Chemical Reaction Calculator displays a balanced equation the structure and all related data for any chemical reaction.

Pin By Tanji On Chemistry Education Chemistry Lessons Chemistry Classroom Chemistry Basics
Both reciprocity and net change are illustrated below in examples of the three most common types of oxidation-reduction Read More.
. Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential as a measurable and quantitative phenomenon and identifiable chemical change with either electrical potential as an outcome of a particular chemical change or vice versaThese reactions involve electrons moving between electrodes via an electronically. 315 The Anatomy of an OxidationReduction Reaction. In any oxidation reaction a reciprocal reduction occurs and 2 they involve a characteristic net chemical changeie an atom or electron goes from one unit of matter to another.
Everyone has seen the reddish color of iron rust which is the end product of the reaction of iron with oxygen as shown in Figure 3151. On the other hand Fe would be written as the oxidation half-reaction when compared to any other element on this table. Factors Controlling Soil Reaction 4.
The growth and activity of. This means that Li would be written as the reduction half-reaction when compared to any other element in this table. Oxidationreduction reactions redox reactions are those reactions in which electrons are transferred from one type of particle to another.
One of the outstanding physiological characteristics of the soil solution is its reaction. Types of Soil Reaction. In this article we will discuss about- 1.
Soil reaction influences many physical and chemical properties of soil. A The position of hydrogen in the periodic table Hydrogen is first element in the periodic table. An oxidizing agent also known as an oxidant oxidizer electron recipient or electron acceptor is a substance in a redox chemical reaction that gains or acceptsreceives an electron from a reducing agent called the reductant reducer or electron donorIn other words an oxidizer is any substance that oxidizes another substance.
STUDYQUERIESs online chemical reaction calculator tool makes calculations faster and easier displaying the equilibrium constant in a fraction of a second. In the table provided the most easily reduced element is Li and the most easily oxidized is iron. It has an atomic number l and an atomic mass of 100794 amu occupying group IA.
Types of Soil Reaction 2. Selina ICSE Solutions for Class 9 Chemistry Chapter 9 Study of the First Element hydrogen.

How To Identify Oxidized And Reduced Element In Redox Reaction With Examples And Problems Youtube

Chemistry Redox Reactions Physics And Mathematics

Electricity In 2020 Science Notes Electricity Elementary Science
No comments for "Which Element Is Oxidized in the Reaction Below"
Post a Comment